HCOOCH CH₂ H₂O is a multifunctional organic compound that combines ester, carbonyl, and hydroxyl groups in its structure. It is highly polar, soluble in water and alcohols, and capable of forming strong hydrogen bonds, which makes it valuable in chemical, pharmaceutical, and green technology applications.
What is HCOOCH CH₂ H₂O? In simple terms, it is a reactive organic molecule with ester and hydroxyl features, offering reactivity for polymerization, esterification, and condensation reactions. Its adaptability allows it to serve as a precursor, solubilizer, and catalyst intermediate, while its eco-friendly behavior positions it as a promising candidate for sustainable chemistry.
Structural Interpretation
The structure of HCOOCH CH₂ H₂O can be interpreted in more than one way. One possibility is that it exists as an ester-hydrate, in which a formate ester backbone is linked with a hydroxyl or water functionality. This would allow it to act both as a stable compound and as a reactive intermediate under certain conditions. Another interpretation is that the formula represents a reaction notation, where ester fragments interact with water through hydrolysis, producing alcohol derivatives. More broadly, the grouping suggests that the compound contains ester, carbonyl, and hydroxyl functionalities within a single system. This unique combination explains the breadth of its chemical behavior and the wide range of applications it could potentially support.
General Properties
Polarity and Solubility
- Highly polar compound due to ester and hydroxyl groups.
- Strong solubility in polar solvents like water and alcohol.
- Limited solubility in hydrocarbons.
Hydrogen Bonding
- Carbonyl oxygen acts as a hydrogen-bond acceptor.
- The hydroxyl group donates hydrogen bonds, aiding in biological interactions and solubility.
Volatility and Stability
- Expected to show moderate volatility, typical of esters.
- Hydroxyl increases the boiling point.
- Likely stable under ambient conditions but decomposes under strong acids, bases, or heat.
Comparative Table: Key Properties
| Property | Likely Behavior of HCOOCH CH₂ H₂O |
| Functional Groups | Ester, carbonyl, hydroxyl |
| Polarity | Highly polar |
| Solubility | High in water and ethanol |
| Volatility | Medium (ester-like) |
| Reactivity | Hydrolysis, esterification, polymerization |
| Biodegradability | Readily biodegradable |
Industrial and Research Applications
Role in Organic Synthesis
In organic synthesis, HCOOCH CH₂ H₂O serves as a versatile intermediate. Its ester group allows it to take part in esterification processes, while the hydroxyl group enables polymer formation and condensation reactions. This dual reactivity increases its value in the preparation of specialty chemicals and advanced intermediates.
Function in Catalysis
In catalysis, HCOOCH CH₂ H₂O shows promise as a hydrogen donor in redox transformations. It could also play a role in CO₂ utilization pathways, which are gaining importance in sustainable chemistry. The combined reactivity of ester and hydroxyl groups enhances its potential in specialty catalytic systems used for fine chemical production.
Pharmaceutical Applications
The pharmaceutical industry could benefit greatly from HCOOCH CH₂ H₂O. Its solubility characteristics allow it to act as a drug solubilizer, improving the bioavailability of poorly soluble drugs. As a precursor for prodrugs, its ester moiety enables controlled release, while the hydroxyl functionality supports modification into drug carriers or polymer-based delivery systems. These features open avenues for innovative therapies and improved drug formulations.
Role in Green Chemistry
Green chemistry values compounds that are biodegradable and derived from renewable sources. HCOOCH CH₂ H₂O fits this model well, since formates can be obtained from biomass or CO₂-based processes. Its use as an eco-friendly solvent or reactive intermediate reduces dependence on petroleum-based chemicals. Beyond solvents, it has potential in adhesives, coatings, and resins, particularly where hydroxyl groups improve cross-linking and bonding performance.
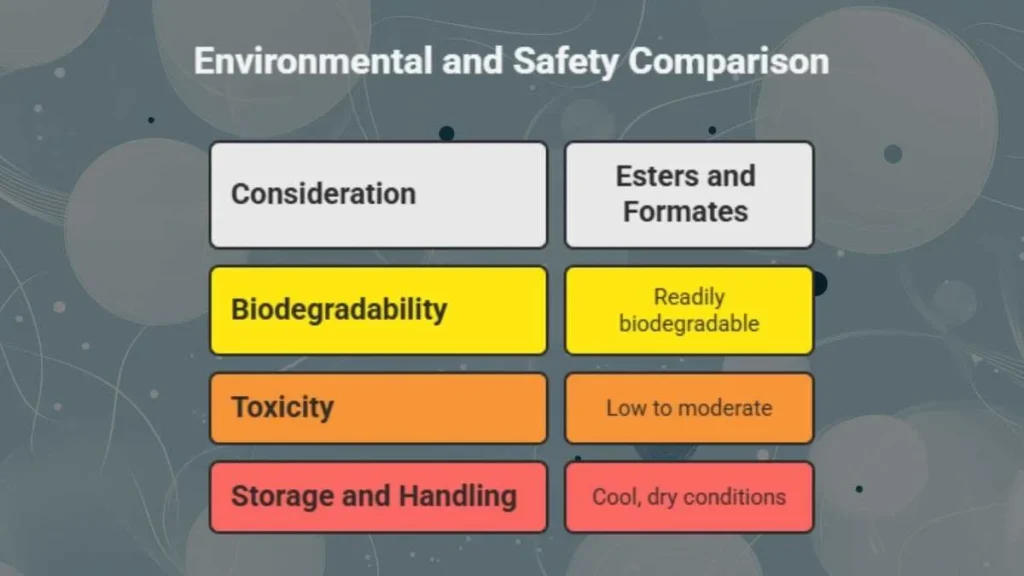
Environmental and Safety Considerations
- Biodegradability
Esters and formates are generally readily biodegradable, reducing long-term environmental persistence.
- Toxicity
Expected to be of low to moderate toxicity, though derivatives may irritate skin or mucous membranes. Safety data sheets for similar formats suggest precautions with handling and inhalation.
- Storage and Handling
Store in cool, dry conditions. Avoid prolonged exposure to heat or strong acids/bases, which may trigger decomposition.
Future Outlook
The future potential of HCOOCH CH₂ H₂O lies in its ability to bridge industrial chemistry with sustainability. Research can focus on designing bio-based solvents that incorporate such multifunctional molecules, replacing conventional petrochemical products. Pharmaceutical scientists may develop new prodrug systems and controlled-release carriers by exploiting their ester-hydroxyl framework. Polymer scientists could use it to design resins and adhesives with improved bonding capabilities. Catalysis researchers might explore its role in hydrogen storage or CO₂ conversion technologies, where multifunctional organic molecules are in demand.
FAQs
Q1: Is HCOOCH CH₂ H₂O naturally occurring?
A: It is not commonly found in nature but can be derived from formate-based synthesis routes.
Q2: Can it replace traditional solvents?
A: Yes, its polarity and biodegradability make it a sustainable alternative to harmful solvents.
Q3: Does it have a distinct odor like other esters?
A: Likely, as most esters exhibit sweet or fruity smells with moderate volatility.
Conclusion
HCOOCH CH₂ H₂O is not merely a chemical formula; it is a conceptual model for multifunctional organic chemistry. By combining ester, carbonyl, and hydroxyl groups in one structure, it offers high solubility, reactivity, and hydrogen bonding, which in turn makes it suitable for a wide range of industrial, pharmaceutical, and environmental applications.
As industries seek solutions that are both high-performing and sustainable, compounds like HCOOCH CH₂ H₂O hold promise for transforming the chemical landscape. Whether as a solubilizer, a polymer precursor, or a green solvent candidate, its role in shaping future chemistry is both valuable and inspiring.








